Vaccine Timeline
Educational Material, Fair Access, IP reform, Vaccine Equity, Access and Allocation
Timeline of key South Africa COVID-19 related developments and Health Justice Initiative’s engagement with government on vaccine access for South Africa
This Vaccine Access timeline has been compiled by the Health Justice Initiative (HJI) with information currently in the public domain and contains approximate dates in some cases, references to public documents, media statements as well as declarations under oath (court papers). It contains certain, but not all key dates related to major international and local developments. It is not meant to be a comprehensive timeline of all of the global or local set of events related to Covid-19. It is however intended to illustrate the global and local context against which the HJI has sought to obtain information and answers to the pressing issue of timely vaccine access for all people in South Africa.
Note: Since the HJI’s launch in mid-2020, we have intervened as a friend of the court with Open Secrets (amicus) in two pandemic ‘price gouging’ matters in the Competition Appeal Court (Dischem/Babelegi); and referred a pricing inquiry with Ezintsha at WITS University on certain available treatments for severe cases of Covid-19 to the Competition Commission.
More information about these other matters is available at Health Justice Initiative.
For any material omissions or errors regarding this timeline, please notify us by email: [email protected]
Updated on 4 April 2022
___________________
Other resources:
Médecins Sans Frontières
PrEP4All
Public Citizen
I-MAK
KEI
O’Neill Institute for National and Global Health Law
The People’s Vaccine
Global Justice Now
Free the Vaccine
Amnesty International
Oxfam
Health GAP
Third World Network
South Centre
UNICEF
2020
- 30 January 2020
-
5 March 2020
First official case of COVID-19 reported in South Africa
National Institute for Communicable Diseases 11 March 2020.
- 5 March 2020
- 15 March 2020
-
25 March 2020
COVID-19 Ministerial Advisory Committee (MAC) established by the National Minister of Health (Dr Zweli Mkhize) and Chaired by Professor Salim Abdool Karim
-
26 March 2020 (midnight)
3-week ‘Lockdown Level 5’ commenced in South Africa
Statement by President Cyril Ramapohosa on the escalation of measures to combat COVID-19 epidemic.
- 30 March 2020
- 1 May 2020
-
14 May 2020
New Director-General of Health for the National Department of Health appointed: Dr Sandile Buthelezi
-
14 May 2020
More than 140 world leaders, experts and elders make an unprecedented call for guarantees that 'COVID-19 vaccines, diagnostics, tests and treatments will be provided free of charge to everyone, everywhere ' - including the President of South Africa, Cyril Ramaphosa, the Prime Minister of Pakistan, Imran Khan, the President of the Republic of Senegal, Macky Sall and the President of the Republic of Ghana, Nana Addo Dankwa Akufo-Addo.
Global leaders sign an open letter calling on all governments to unite behind a people’s vaccine against COVID-19.
- June 2020
- 4 June 2020
-
Mid-June 2020
Globally, the ‘Peoples Vaccine Campaign’ and the ‘Free the Vaccine’ initiative are launched . It calls for ‘Everyone, everywhere, who needs it, must get a safe and effective vaccine’. And that: ‘COVID-19 diagnostic tools, treatments, and vaccines must be available to everyone everywhere, free at the point-of-delivery’
Access the link here for more information.
-
16 June 2020
Multi-sectoral Ministerial Advisory Committee (MAC) for Social and Behavioral Change established by the National Minister of Health
-
July 2020
Health Justice Initiative (HJI) - a new NPO, is launched in South Africa
- 1 July 2020
-
July 2020
Department of Health stated that it initiated discussions with certain vaccine manufacturers
Director-General of Health (Dr Sandile Buthelezi) : Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 108. Access the link here for more information.
- 17 July 2020
-
24 July 2020
The National Department of Health stated that its engagement with Pfizer commenced
Director-General of Health, Answering affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 141. Access the link here for more information.
- 27 August 2020
- September 2020
- September 2020
-
3 September 2020
COVAX launched
COVAX is one of three pillars of the Access to COVID-19 Tools (ACT) Accelerator, which was launched in April 2020 by the World Health Organization (WHO), the European Commission and France in response to COVID-19 – a ‘global risk-sharing mechanism for pooled procurement and equitable distribution of COVID-19 vaccines’. Access the link here for more information.
-
4 September 2020
The National Department of Health stated that it commenced negotiations with Johnson & Johnson (followed by ‘further extensive engagements in January 2021)
Director-General of Health, Answering affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 135. Access the link here for more information.
-
14 September 2020
The National Department of Health stated that it first engaged the Serum Institute of India
Director-General of Health, Answering affidavit Afriforum & Solidarity v Minister of Health and 16 others, para 125. Access the link here for more information.
-
15 September 2020
The National Minister of Health announced changes to the members of the COVID-19 Scientific Ministerial Advisory Committee (MAC): 'Committee members were evaluated and changed to include more experts outside of the biomedicine sector such as ethicists, social scientists, and community leaders'
The Ministerial Advisory Committee is as a result co-chaired by Professors Salim Abdool Karim and Professor Marian Jacobs with Dr Nhlanhla Mkhize, Professor Barry Schoub and Dr Portia Jordan as joint vice-chairs. Access the link here for more information.
- 15 September 2020
- 29 September 2020
- 1 October 2020
-
2 October 2020
TRIPS Waiver Proposal
India and South Africa jointly and formally proposed that the World Trade Organization (WTO) permit all countries to choose to neither grant nor enforce patents and other intellectual property (IP) related to COVID-19 drugs, vaccines, diagnostics and other technologies for the duration of the pandemic, until global herd immunity is achieved (not just for patents, but for trade secrets, copyrights, and industrial design)
To date, the waiver is co-sponsored by 62 countries (including Eswatini, Kenya, Mozambique, Pakistan, Mongolia, Venezuela, Bolivia, Zimbabwe and Egypt) and supported by at least 100 countries, but continues to be opposed by a small group of member states including: Japan, Brazil, Australia, EU, UK, US, Canada, Norway, Switzerland, Ecuador, El Salvador
- 14 October 2020
-
October 2020
The National Department of Health stated that its preliminary engagements with the vaccine manufacturers of Sputnik V commenced
Director-General of Health, Answering affidavit Afriforum & Solidarity v Minister of Health and 16 others, para 155.1. Access the link here for more information.
- 2 November 2020
-
9 November 2020
Pfizer/BioNtech announced top-level phase III trial results via a press release
- Director-General of Health, Answering affidavit Afriforum & Solidarity v Minister of Health and 16 others, para 155.2. Access the link here for more information.
-
16 November 2020
HJI sent Letter 1 to the Ministers of Cooperative Governance & Traditional Affairs and Health; and the National Disaster Management Centre (DMC)
Letter 1 requested information about the details of the development of a plan to ensure affordable access to and equitable allocation of a COVID-19 vaccine and in particular, requested answers to the following questions:
- Has the DMC developed Vaccine Access and Allocation plans?
- Are there plans to budget and prioritise vulnerable groups?
- Do key institutional role-players have Vaccine Access and Allocation Plans, and if these do not exist, please provide timelines for their publication?
- 16 November 2020
-
16 November 2020
The National Department of Health stated that on 16 November 2020 it began engaging with Moderna and that on 21 December 2020 the National Minister of Health met with Moderna executives
Director-General of Health, Answering affidavit Afriforum & Solidarity v Minister of Health and 16 others, para 151. Access the link here for more information.
- 2 December 2020
-
2 December 2020
HJI sent Letter 2 to the Ministers of Cooperative Governance & Traditional Affairs and Health; and the National Disaster Management Centre (DMC) due to no response being received in respect of Letter 1
Urgent questions about Vaccine Access and Allocation Plans are repeated, as per Letter 1
A deadline for a response is extended to 8 December 2020
-
8 December 2020
AstraZeneca/Oxford University phase III trial peer-reviewed results released
Director-General of Health, Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 123. Access the link here for more information.
-
8 December 2020
Dr Mmaphaka Tau, head of the South African National Disaster Management Centre (DMC) responded to HJI Letters 1 and 2
‘The National Department of Health is the organ of state responsible for vaccine related matters.’
- 9 December 2020
-
10 December 2020
South Africa and COVAX
South Africa formally registers to participate via the COVAX facility as a middle income country
Director-General of Health, Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 101. Access the link here for more information.
-
11 December 2020:
The U.S. Food and Drug Administration issued its first emergency use authorization (EUA) for a Covid-19 vaccine to Pfizer /BioNTech- for individuals 16 years of age and older
Note: A few days prior to the advisory panel meeting of December 10, the Food and Drug Administration released their independent assessment of the data which becomes the first publication of detailed data beyond Pfizer’s press releases. Access the link here for more information.
-
15 December 2020
HJI sent Letter 3
- Letters 1 and 2 are unanswered by the National Department of Health
- HJI suggests an ‘urgent multi-stakeholder engagement’ to develop an appropriate and urgent Vaccine Access and Equitable Allocation Plan for South Africa
- Letter 3
-
18 December 2020
The U.S. Food and Drug Administration issued an emergency use authorisation (EUA) for a second Covid-19 vaccine to Moderna - for individuals 18 years of age and older
Note: A few days prior to the advisory panel meeting of December 17, the Food and Drug Administration released their independent assessment of the data which becomes the first publication of detailed data beyond Moderna’s press releases. Access the link here for more information.
- This is followed by authorisation in Canada on 23 December 2020, and in Israel on 4 January 2021.
- As at 28 March 2021 Moderna did not file a regulatory approval submission/ dossier with the South African Health Products Regulatory Authority
- 21 December 2020
-
21 December 2020
The South African Solidarity Fund made a down payment of approximately $19.2 million (ZAR 283 million) for South Africa to participate in the COVAX facility
Director-General of Health, Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 102. Access the link here for more information.
- 30 December 2020/ 3 January 2021
2021
- 2 January 2021
-
3 January 2021
South Africa’s ‘Covid-19 vaccine rollout strategy’ is announced at a press briefing by the National Minister of Health and others on national television
Minister Zweli Mkhize Public Briefing Statement South Africa’s COVID-19 Vaccine Strategy.
- 6 January 2021
-
6 January 2021
Department of Health applied to the National Treasury for permission for a deviation to conclude agreements with vaccine manufacturers
Director-General of Health, Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 109, available. Access the link here for more information.
-
7 January 2021
Pfizer submitted its regulatory application/dossier to the South African Health Products Regulatory Authority
- 7 January 2021
-
7 January 2021
The National Minister of Health announced an order of the AstraZeneca vaccine for all healthcare workers in South Africa
Minister Mkhize says SA will receive first batch of COVID-19 vaccine in January 2021.
- 7 January 2021
-
7 January 2021
Term-sheet between the National Department of Health and the Serum Institute of India signed for 1.5 million doses, followed by a purchase agreement, on 18 January 2021
Director-General of Health, Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 123-8. Access the link here for more information.
- 7 January 2021
- 8 January 2021
- 11 January 2021
-
12 January 2021
The People’s Vaccine Campaign of South Africa (PVC-SA) is formed and issued a national Call to Action
Towards a People’s Vaccine Campaign – a Call to Action.
- 13 January 2021
-
15 January 2021
The National Department of Health stated that a term-sheet with Pfizer was signed for 20 million doses with the purchase agreement pending
Director-General of Health, Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 145. Access the link here for more information.
- 18 January 2021
-
19 January 2021
The Africa Medical Supplies Platform (AMSP), on behalf of the Africa Centres for Disease Control and Prevention (Africa CDC), commenced a vaccine pre-order programme for all AU Member States
According to the AU, the African Export-Import Bank (Afreximbank) will ‘facilitate payments by providing advance procurement commitment guarantees of up to US$2 billion to the manufacturers on behalf of the Member States’.
-
Mid-late January 2021
Johnson & Johnson dossier for purposes of a rolling review is lodged for consideration by the South African Health Products Regulatory Authority (approved on 1 April 2021)
- Mid-late January 2021
-
29 January 2021
Johnson & Johnson phase III trial results released globally via a press statement
- 29 January 2021
- 31 January 2021
- 31 January 2021
-
February 2021
The National Department of Health indicated that it ‘had multiple discussions with the Chinese vaccine manufacturers (Sinovac and Sinopharm), which were facilitated via the Chinese embassy’ (exact date/s unclear)
Director-General of Health, Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 155.2. Access the link here for more information.
-
1 February 2021
HJI, via its legal representatives, Power Singh Incorporated (PSI), sent a Letter of Demand (Letter 4) to the South African government including the Presidency, National Command Centre and the Speaker of Parliament, requesting a response by 12 February 2021
- 1 February 2021
- 2 February 2021
- 2 February 2021
-
3 February 2021
COVAX issued its first interim supply and distribution forecast, indicating 2.976 million AstraZeneca and just under 177 000 Pfizer vaccines for Q1 delivery, for South Africa
Note: On 3 March 2021, an updated forecast was issued as follows:
‘117 000’ – Pfizer
‘2 426 000’ – AstraZeneca*
* Footnote 4 stated: ‘Use of allocation under discussion; potential to be reallocated’
Access the link here for more information.
- February - March 2021
- 4 February 2021
-
7 February 2021
Results of the AstraZeneca/Oxford University vaccine against the 501Y.V2 variant first discovered in South Africa is announced at a national press briefing (convened at short notice) with the National Minister of Health, Professor Shabbir Madhi, Professor Glenda Gray, Professor Barry Schoub and the National Minister of Health, Dr Zweli Mkhize
- A decision to pause the use of the vaccine for health care workers in South Africa is announced at that briefing.
- The National Minister of Health stated that ‘the scientists’ will meet in the ‘next day’ to provide formal advice to government on the possible next steps regarding the vaccines.
- Shortly after the press briefing, at the State of Nation Address 2021, the National Minister of Health indicates to journalists that the batch could be used for a ‘study trial’ but it is subsequently sold to the AU.
- To date, no information has been provided to the public on the details and nature of ‘the scientists’ advice – and it is unknown on whose written scientific advice this decision was made, which has recently come under criticism.
Access the link here for more information.
-
10 February 2021
The Minister of Health briefed the Portfolio Committee on Health on the latest developments in relation to the AstraZeneca vaccine.
He also stated that in respect of Sinopharm, there had been ‘offers from China’ of doses; and that the said regulatory dossier was lodged with the South African Health Products Regulatory Authority (SAHPRA). Access the link here for more information.
- 15 February 2021
-
15 February 2021
The National Department of Health indicated that the vaccine doses from the Serum Institute of India will be offered to the African Union (for AVATT)
Director-General of Health, Answering Affidavit Afriforum & Solidarity v Minister of Health and 16 Others, para 131. Access the link here for more information.
- 15 February 2021 (to 5 March 2021)
- 17 February 2021
- 18 February 2021
-
22 February 2021
Vaccine procurement (and allocation case) brought by Afriforum and Solidarity against National Minister of Health and Others - HJI intervened as Amicus (friend of the court)
The HJI filed an application to intervene as amicus curiae in Solidarity and Afriforum’s urgent application to the Pretoria High Court related to ‘COVID-19 Vaccine Acquisition in South Africa’.
The HJI also sought to introduce public health and expert evidence on the issue of equitable allocation during a pandemic amidst supply scarcity
- Solidarity and Afriforum, challenged the constitutionality and legality of the Government’s roll-out strategy, as set out in the department’s “COVID-19 response” document of 7 January 2021.
- The HJI brought the application because while Solidarity and Afriforum consented to the admission of HJI, it had not consented to HJI adducing expert evidence and none of the 17 Respondents had indicated their position or consent by then
Note: Solidarity and Afriforum withdrew the case about one week after HJI filed its application to be admitted as a friend of the court and to adduce expert evidence
- 24 February 2021
- 23/24 February 2021
- 25 February 2021
-
27 February 2021
The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for Johnson & Johnson - for use in individuals 18 years of age and older
The briefing document was released a few days prior. Access the link here for more information.
- 27 February 2021
-
2 March 2021
Afriforum and Solidarity formally withdraw its legal case
HJI issued its statement on the withdrawal of the case, noting that ‘due to the formal withdrawal of its case, Afriforum and Solidarity have not responded on record to the expert evidence introduced by the HJI which sought to emphasise the principle of prioritisation and equity in a pandemic’.
-
9 March 2021
HJI requested that the National Department of Health place all Ministerial Advisory Committee Vaccine Advisories (mid-January 2021 onwards) in the public domain
- The Department’s MAC secretariat representative replied that ‘internal processes’ must be followed before it can be published
- The HJI followed-up on 23 March 2021 and since.
- The HJI wrote to the MAC Secretariat on 14 April 2021 asking for same and included a recent opinion written by the Chairperson of the MAC Vaccines in the South African Medical Journal.
- After numerous reminders from the HJI, the Department of Health subsequently published 7 additional MAC advisories.
- HJI sent further correspondence on 30 April 2021 requesting the urgent publication of the MAC Vaccine advisories that relate to the decision to pause the use of the AstraZeneca vaccine.
- 10 March 2021
-
10 - 11 March 2021
World Trade Organization (WTO) member states continue to discuss the TRIPS waiver proposal
South Africa’s opening statement at the meeting is available here.
- 11 March 2021
-
11 March 2021
Statement issued by the UN Secretary-General on the COVID-19 Pandemic
The UN Secretary-General expressed concerns that ‘many low-income countries have not yet received a single dose, while wealthier countries are on track to vaccinating their entire population’.
He lambasted the examples of vaccine nationalism and vaccine hoarding and ‘side deals with manufacturers’ that undermine access for all.
And, he stated that, ‘COVID-19 vaccines must be seen as a global public good. The world needs to unite to produce and distribute sufficient vaccines for all, which means at least doubling manufacturing capacity around the world’
-
11 March 2021
Director-General of Health responded to HJI’s Letter 1 (dated 16 November 2020) - he addressed some but not all of the questions contained in HJI’s Letters 1-3
The DG stated that the Ministerial Advisory Committee on Covid-19 vaccines (MAC VAC) was appointed by the Minister of Health to ‘provide the strategic framework for access to COVID-19 vaccines’.
Also that ‘the MAC VAC is in the process of developing the COVID-19: Vaccine Strategy. This Strategy will be available on the National Department of Health’s website once it has been finalised’.
-
13 March 2021
Global Day of Action for a People’s Vaccine is launched in the UK, US, India, Europe, New Zealand and parts of Africa
In the UK and US, protest action is held outside the head offices of Pfizer Inc, AstraZeneca, Moderna Inc and Johnson & Johnson
- In South Africa, memoranda are electronically delivered, and in certain cases handed over in person, through protest action by the PVC-SA to: Johnson & Johnson, AstraZeneca, Pfizer Inc, Aspen Pharmacare and Moderna Inc. Access the link here for more information.
- To date, only Johnson & Johnson have formally replied in writing to the PVC-SA Memorandum
- 16 March 2021
- 17 March 2021
-
12 - 17 March 2021
2021 Conference on Retroviruses and Opportunistic Infections (CROI) ‘Declaration for Vaccine Equity’ issued, following the Martin Delaney opening presentation
Started by scientists, public health and legal experts, and community leaders assembled from around the world for the Conference as at 24 March 2021, the Declaration has over 1000 signatories from various scientists, community leaders and public health and human rights specialists.
-
19 March 2021
BioVac (SA company) announced a new deal with the U.S.-based company - ImmunityBio Inc
BioVac will now ‘make raw materials to enable it to join Senegal’s Institut Pasteur de Dakar as one of two companies able to make vaccines from start to finish in sub-Saharan Africa’.
-
23 March 2021
The National Minister of Health confirmed that 1 million doses of the Covishield (Serum Institute of India/ AstraZeneca) Vaccine was sold to the African Union (AU) for 14 countries after the AU's vaccine acquisition team ‘found that they have obtained all regulatory approvals, permits and licenses to rollout the vaccines’
Access the link here for more information.
-
23 March 2021
United Nations Human Rights Council Resolution on Vaccine Equity issued
Resolution A/HRC/46/L.25/Rev.1 UN HRC Resolution urged states to ensure ‘equitable, affordable, timely and universal access for all countries’ to vaccines.
-
23 March 2021
Speaking at an Economic Commission for Africa (ECA), World Health Organisation (WHO) Director-General, Dr Tedros Adhanom Ghebreyesus urged equitable vaccine distribution and for African countries to ratify the African Medicines Agency to regulate manufacturing on the continent
He noted that ‘ultimately Africa needs to be able to meet its own needs for vaccines and other essential products. That means financing local manufacturing capacity, comprehensive regulation and sustainable supply chains’. Access the link here for more information.
-
24 March 2021
Informal meeting on the TRIPS Waiver held (there have been numerous formal and informal meetings and discussions with member states about the waiver proposal since October 2020)
For more information, key dates, analysis and outcomes regarding the TRIPS Waiver see:
-
27 March 2021
The largest private Medical Scheme in the country (Discovery Health) issued a letter to its members
It stated that:
‘the country has secured 31 million doses of vaccines in total, which is sufficient to vaccinate approximately 21 million people (some vaccines require double doses). This comprises 11 million doses of the J&J vaccine and 20 million doses of the Pfizer-BioNTech vaccine, which are both highly effective and suitable. In addition, there are possibly 20 million doses of the J&J vaccine that are being negotiated. This would be sufficient to cover more than the 37 million adults in South Africa, exceeding the population herd immunity target of 29 million people’
-
28 March 2021
Mass Vaccine Roll Out Announcement
The National Department of Health announced that it ‘is working in partnership with the private sector to undertake a mass vaccine roll out when Phase 2 gets underway’ from May – October 2021
According to the National Department of Health and Government, there will be three phases of the Vaccination Roll-Out Programme:
-
- Phase 1: February – April 2021 (‘1.5 million healthcare workers’)
- Phase 2: May – October 2021 (‘13.3 million vulnerable groups, essential workers and occupational health and safety stream’)
- Phase 3: November 2021 – February 2022 (approximately 22 million people, not covered in Phases 1 and 2)
South Africa’s #COVID19 Vaccination roll-out programme will take place in 3 phases #VaccineforSouthAfrica pic.twitter.com/zBxeiJCPhe
— South African Government (@GovernmentZA) March 28, 2021 -
- 29 March 2021
-
30 March 2021
The Minister of Health informed Parliament that:
- ‘South Africa would acquire 31 million doses’ of the Johnson & Johnson vaccine and that the Health Department expected to vaccinate about 41.5 million people before March 2022.
- He also stated that the roll out prioritisation plan would be as follows:
- Phase 1 (17 February – 17 May), targeting 608 295 healthcare workers
- Phase 2A (17 May – July 31), targeting 5 449 980 people over the age of 60
- Phase 2B (August – 31 October), targeting 12 900 160 people over the age of 40, and prioritising those with co-morbidities and workers in high-risk settings
- Phase 3 (November – 28 February 2022), targeting 22 600 640 people.
Access the link here for more information.
- 31 March 2021
-
9 April 2021
The HJI wrote to the CEO of the South African Health Products Regulatory Authority (SAHPRA) requesting certain measures to increase transparency of SAHPRA processes and decision-making for Covid-19, especially for vaccine approvals. SAHPRA responded on 5 May noting that their legal team is considering the request
-
13 April 2021
The use of the Johnson & Johnson vaccine was paused in the US by the FDA, and in Europe by Johnson & Johnson following reports of six women who had developed blood clots out of out of 7 million vaccinated Americans
-
- On the basis of the FDA decision, the Minster of Health announced that South Africa would pause the Sisonke trial/study too. Access the link here for more information.
- The Department of Co-operative Governance and Traditional affairs releases a draft set of regulations entitled COVID-19 Vaccine Injury No-Fault Compensation Scheme .
-
-
- The public was invited to comment by 19 April 2021 (shortened time period).
-
- The HJI submitted a short submission which was endorsed by 14 organisations and 17 individuals and available here.
-
-
- 14 April 2021
- 14 April 2021
-
21 April 2021
The South African Minister of Trade, Industry and Competition called for a “COVID New Deal” to increase vaccine supplies
- This includes sharing of know-how and technology, passing the TRIPS waiver and developing processes for future pandemics, ensuring transparency of contracts, and a commitment by all countries to reject vaccine nationalism. Access the link here for more information.
-
21 April 2021
The People's Vaccine Alliance hosted a global discussion and invited pharmaceutical company CEOs, not one CEO attended or responded to the invitation
Access the link here to watch the webinar.
- 23 April 2021
- 23 April 2021
-
28 April 2021
The National Department of Health briefed Parliament and indicated that it was expecting ‘31 million’ vaccines from Johnson & Johnson and has concluded a contract with Pfizer.
It also said it was “prioritising senior citizens, those over 40 years who had comorbidities and workers on the frontline that included teachers, police, industrial workers and community workers”.
- 30 April 2021
-
5 May 2021
The US Government announced partial support for the TRIPS Waiver proposal and for text based negotiations.
The announcement was made by United States Trade Representative Katherine Tai. It noted that the US government’s support for a temporary waiver is limited to vaccines (not diagnostics, therapeutics and Personal Protective Equipment (PPE)). Access the link here for more information.
- 6 May 2021
- 7 - 10 May 2021
- 12 May 2021
- 15 May 2021
- 17 May 2021
- 17 May 2021
- 18 May 2021
- 21 May 2021
-
27 May 2021
HJI wrote to the Minister of Health, the CEO of the South African Health Products Regulatory Authority and the CEO of the South African Medical Research Council to request information on the prioritisation of “elite athletes” for vaccination under the Sisonke study
- 27 May 2021
-
29 May 2021
CSOs in South Africa and India write to the Ministers of Trade, Industry and Competition and Commerce and Industry in South Africa and India respectively, on the ‘Launch of Negotiations on the TRIPS Waiver at the WTO’ setting out the minimum demands of civil society
-
31 May 2021
Government announced that by end May 2021, at least 970 448 people had been vaccinated in South Africa including through the Sisonke study
479 768 people were vaccinated under Sisonke, and 490 680 people were vaccinated with the first dose of a Pfizer vaccine under Phase Two of the National Roll-out. Access the link here for more information.
- 1 June 2021
- 4 June 2021
-
4 June 2021
The European Union submitted communication IP/C/W/680 to the Council for TRIPS titled “Urgent Trade Policy responses to the COVID-19 crisis: Intellectual Property”.
This was followed by another communication on 18 June 2021 proposing a ‘declaration on the TRIPS Agreement and Public Health in the circumstances of a pandemic’ (IP/C/W/681).
- 7 June 2021
- 8 June 2021
- 10 June 2021
-
10 June 2021
The European Parliament voted to pass a critical resolution on meeting the global COVID-19 challenge and to support text-based negotiations on the TRIPS waiver proposal.
The resolution calls for revisiting the global framework for intellectual property (IP) rights for future pandemics; and supports proactive, constructive and text-based negotiations for a temporary waiver of the WTO TRIPS Agreement, aiming to enhance global access to affordable COVID-19-related medical products and to address global production constraints and supply shortages.
-
11 - 13 June 2021
The G7 summit was held in Cornwall in the UK where G7 leaders discussed its response to the pandemic and to climate change. The governments of South Africa, India, South Korea and Australia were also invited to attend the meeting.
Prior to the meeting, the People’s Vaccine movement pointed out that 8 people have died of COVID every minute since the last G7 meeting that was held in February 2020. And that people living in G7 countries are 77 times more likely to be offered a vaccine than those living in the poorest countries in the world. Access the link here and here for more information.
- 12 June 2021
-
13 June 2021
The G7 pledged 870 million COVID-19 vaccine doses, of which at least half would be delivered by the end of 2021, despite initially announcing a pledge of 1 billion vaccine doses.
- 13 June 2021
- 17 June 2021
- 19 June 2021
-
21 June 2021
The French Embassy in South Africa announced its own vaccination campaign for French nationals over the age of 55 years old in South Africa and Lesotho, using its own batch of imported vaccines. Some media reports indicate that other EU countries are doing the same.
- 21 June 2021
-
22 June 2021
HJI wrote to the Acting Minister of Health and relevant Information Officers requesting the voluntary and public disclosure of all
- COVID-19 vaccine procurement and supply contracts.
- MAC Advisories for 2021.
- Correspondence with SAHPRA and any research body or ethics committee regarding the prioritisation of ‘elite athletes’ for vaccination under Sisonke/other.
- 23 June 2021
- 23 June 2021
- 24 June 2021
- 26 June 2021
- 27 June 2021
-
27 June 2021
The President stated that South Africa “received an additional 1.2 million doses of the Johnson & Johnson vaccine and also 1.4 million doses of the Pfizer vaccine through the COVAX facility”.
- And that:
- Over 950,000 health care workers had been vaccinated
- The registration and vaccination of the over 60 year old group was underway with certain challenges in reaching 5 million people in that group
- 50 to 59 year olds would begin registration on 1 July, with vaccination commencing on 15 July
- Vaccination of people working in the basic education sector was underway, using J&J (184 000 by then)
- Police and other security personnel would commence vaccination on 5 July
- Workplace programmes in economic sectors such as mining, manufacturing, and the taxi industry would also commence
- And that:
- 28 June 2021
-
28 June 2021
The African Union Commission and the Africa Centres for Disease Control and Prevention (Africa CDC) issued a ‘Statement on Covishield and the European Union (EU) Digital COVID Certificate “Green Pass"’. It noted with concern the applicability of the EU Digital COVID Certificate “Green Pass” for only certain different COVID-19 vaccines.
Countries that receive COVAX vaccines are particularly disadvantaged.
- 29 June 2021
-
29 June 2021
Civil society groups urged the European Commission and EU Member States not to block the TRIPS waiver at the WTO
Over 200 CSO’s from around the world write an open letter to the European Commission and EU Member States requesting that they withdraw parallel EU proposals that do not advance timely, equitable global access to vaccines, treatments, diagnostics, and other COVID-19 health technologies and goods.
- 30 June 2021
-
July 2021
HJI turned 1 year old!
-
1 July 2021
SAHPRA issued a Media release on the approval process of Covid-19 vaccines
Media release on the approval process of Covid-19 vaccines.
-
1 July 2021
SAMRC data showed that 94% of Covid-19 infections in healthcare worker after Johnson & Johnson vaccines are mild
A total of 479 768 healthcare workers received the Johnson & Johnson vaccine as part of the Sisonke programme.
- 1 July 2021
- 2 July 2021
- 3 July 2021
-
3 July 2021
The South African Health Products Regulatory Authority (SAHPRA) authorised the CoronaVac COVID-19 vaccine with conditions
-
- The authorisation was done in terms of Section 21 of the Medicines and Related Substance Act 101 of 1965.
- The vaccine is manufactured by Sinovac Life Sciences Co, and imported by Curanto Pharma (Pty) Ltd.
-
-
4 July 2021
Department of Health released a Circular on the vaccination of ‘special groups’ including Ministers, Premiers and MECs. After a public outcry, the circular was withdrawn on 6 July on the basis that it was not authorised; issued due to a ‘lack of sleep’ in ‘error’ and re-issued in part on 29 July.
Access the Original Circular here.
- 6 July 2021
- 6 July 2021
- 6 July 2021
- 7 July 2021
- 9-13 July 2021
-
11 July 2021
Statement by President Cyril Ramaphosa on progress in the national effort to contain the Covid-19 pandemic.
“To date, over 4.2 million people in South Africa have received a vaccine dose…We are currently vaccinating both the 60+ and 50+ age groups. From this coming Thursday, the 15th of July, those over 35 years of age will be able to register on the Electronic Vaccination Data System. On the 1st of August we hope to commence with vaccinations for the 35+ group.Programmes are underway to vaccinate essential workers, starting with basic education, the police and the defense force
- 13 July 2021
- 15 July 2021
- 16 July 2021
- 16 July 2021
-
19 - 26 July 2021
Following various correspondence to government and other statutory bodies (see other timeline entries) HJI lodged six PAIA (access to information) requests for information relating to:
- All vaccine contracts and agreements with vaccine manufacturers / others entered into with the SA government
- All MAC and VMAC Advisories and other details related to the experts advising government, from January 2021
- In response, the Department of Health published several MAC advisories on 23 and 26 July, respectively
See the link here for more information.
- Information and documentation related to the prioritisation framework; comorbidities; decision to pause AstraZeneca and approval for professional athletes, sports people, sport coaches, and sports administrators from different sporting codes as well as artists and musicians in South Africa to commence vaccination under the Sisonke programme and/or ahead of the general population during March – June.
- 20 July 2021
-
20 July 2021
Justice Moseneke Released his Report for the IEC Inquiry into Ensuring Free and Fair Elections during Covid-19
Justice Moseneke recommended that local elections scheduled for October 2021 be deferred (but not later than February 2022) and issued a Report with detailed findings:
- The report references HJI and PHM-SA’s submission (28 June 2021) – commencing at paragraph 157 of the Report.
- 20 July 2021
- 21 July 2021
- 21 July 2021
- 23 July 2021
- 25 July 2021
- 26 July 2021
-
26 July 2021
Aspen issued a press release indicating that the FIRST supplies to South Africa of the Johnson & Johnson COVID-19 vaccines would be ‘released to Johnson & Johnson’ for the vaccine programme.
It also stated that some of these ‘batches will be made available through the African Vaccine Acquisition Task Team/African Union platform’.
- 27 - 28 July 2021
- 27 July 2021
-
27 July 2021
The EU restated its opposition to the SA-India TRIPS waiver proposal
-
- The French embassy in SA announced that 1 272 French citizens and their family dependents in SA and Lesotho had been vaccinated using French government supplies of the Janssen vaccine.
- The criteria for vaccination have now been changed to include citizens of 18 years and older.
Access the link here for more information.
-
-
28 July 2021
HJI Press Statement ‘Transparency and Secrecy do not go hand in hand in a pandemic’ issued
- The HJI announced that several formal access to information (PAIA) requests had been lodged in previous weeks and with various government departments and statutory bodies.
- The access to information filings requested the disclosure of all vaccine procurement and distribution contracts, all MAC/ VMAC advisories and related expert advice, and all relevant information on the regulatory approval/s for the prioritisation of ‘special groups’.
-
28 July 2021
The Western Cape Health Department stated that the demand for vaccines exceeded the supplies that were available in the province at the time, and media reports indicated that health officials stated that there were not enough supplies in the system due to delays in deliveries on the part of Johnson & Johnson.
-
29 July - (see also 4 July above*)
The Department of Health reissued a slightly amended ‘Special Groups Circular’
It provides dispensation for “special groups and individuals” to apply to receive a vaccine ahead of their age cohort (business people, sports people, people who need to study abroad and seeking medical care abroad) but makes no provision for people with comorbidities and undocumented persons.
-
29 July 2021
The national Department of Health responded to HJI on access to information requests on vaccine contracts indicating that it will notify all manufacturers of requests and invite them to make oral and written submissions before making a decision.
-
30 July 2021
The People’s Vaccine Campaign released a new report ‘The Great Vaccine Robbery’ showing that the cost of vaccinating the world against COVID-19 could be at least five times cheaper if pharmaceutical companies did not profiteer from monopolies on COVID-19 vaccines
Access the link here for more information.
- 30 July 2021
-
31 July 2021
Acting Health Minister records delivery of US donated COVID-19 vaccines (Pfizer)
The donation is for 5.6 million doses.
-
End July 2021
Total Number of Vaccines Administered in South Africa
- J&J (Sisonke) – 1 dose: 485 256 health workers (fully vaccinated)
- J&J – 1 dose: 1 048 206 people (fully vaccinated)
- Pfizer Dose 1: 4 570 135 people
- Pfizer Dose 1 and Dose 2: 1 440 942 people (fully vaccinated)
- August 2021
- 3 August 2021
- 3 August 2021
- 4 August 2021
- 5 August 2021
-
6 August 2021
The HJI responded to the Department of Health’s correspondence on the HJI’s PAIA (access to information) requests. The HJI noted the Department’s intention to consult with ‘vaccine manufacturers and distributors’, and granted an extension to 25 August 2021
- 6 August 2021
- 8 August 2021
- 11 August 2021
- 12 August 2021
-
12 August 2021
The HJI presented its submission on the Copyright Amendment Bill to Parliament with a focus on Covid-19 and the ‘right to repair’.
Access the written submission here.
- 14 August 2021
- 16 August 2021
-
16 August 2021
The New York Times (NYT) revealed that vaccines filled and finished by Aspen in South Africa were being exported to Europe while Africa waited for supplies and as South Africa in particular was entering its third wave.
The NYT also reported that:
- It had seen a copy of the contract between Johnson & Johnson and the South African government which gave Johnson & Johnson the right to export on an unlimited basis.
- At least 32 million vaccines were exported from South Africa to the EU in the first half of 2021.
- Access the link here for more information.
Note: Due to the story in the NYT, exports have since been halted with an undertaking by Johnson and Johnson that all Aspen filled and finished vaccines will remain in Africa*.
- 16 August 2021
-
18 August 2021
The Director-General of the WHO Dr Tedros Grebreysus reacted to the news that Johnson & Johnson exported vaccines to the EU from South Africa.
He indicated that he was ‘stunned by the news that J&J vaccines filled and finished in South Africa are leaving the continent and going to Europe, where virtually all adults have been offered vaccines at this point’. Access the link here for more information.
- 18 August 2021
- 18 August 2021
-
19 August 2021
Medicine and health access groups and activists reacted to reports that Johnson & Johnson exported vaccines from South Africa to the European Union
Per media reports, the European Union Commission announced that the exports were ‘temporary’. Access the link here for more information.
- 19 August 2021
- 23 August 2021
- 26 August 2021
-
End August 2021
Total Number of Vaccines Administered in South Africa:
- Total Vaccines administered: 12 568 525
- People fully vaccinated with Johnson & Johnson vaccine: 2 834 953
- People fully vaccinated with Pfizer vaccine: 3 150 340
- People who received 1st dose of Pfizer vaccine: 6 583 232
- 15% of adults fully vaccinated: 5 985 293
-
2 September 2021
Reuters reported that the African envoy for vaccines stated in a press briefing that Johnson & Johnson had agreed to temporarily pause exporting filled and finished vaccines from South Africa to Europe, following on from a New York Times expose in mid-August 2021
- 2 September 2021
- 7 September 2021
- 8 September 2021
-
8 September 2021
The Director-General of the WHO criticised countries from the global north for hoarding vaccines and vaccine manufacturers for not sharing knowledge and expanding access
He stated that:
“Almost 90% of high-income countries have now reached the 10% target, and more than 70% have reached the 40% target. Not a single low-income country has reached either target. Yesterday, the International Federation of Pharmaceutical Manufacturers Association said that G7 countries now have enough vaccines for all their adults and teenagers, and to offer booster doses to at-risk groups, and that manufacturing scale-up should now shift to delivering global vaccine equity, including dose sharing. When I read this, I was appalled. In reality, manufacturers and high-income countries have long had the capacity to not only vaccinate their own priority groups, but to simultaneously support the vaccination of those same groups in all countries. I will not stay silent when the companies and countries that control the global supply of vaccines think the world’s poor should be satisfied with leftovers.”
He also called for an extension to the moratorium on booster shots:
“A month ago, I called for a global moratorium on booster doses at least until the end of September, to prioritise vaccinating the most at-risk people around the world who are yet to receive their first dose. There has been little change in the global situation since then, so today I am calling for an extension of the moratorium until at least the end of the year, to enable every country to vaccinate at least 40 percent of its population.”
- 8 September 2021
- 9 September 2021
- 14 September 2021
- 14 September 2021
-
9 - 15 September 2021
Pursuant to requests for access to information related to all expert and Ministerial Advisory Committee (MAC) advice including on prioritisation of certain groups, and all vaccine related and manufacturer contracts, which various statutory bodies and the department of health did not respond / accede to:
The HJI filed several internal appeals using the Promotion of Access to Information Act (PAIA).
- The HJI informed the National Department of Health and relevant statutory bodies, that should the appeals not result in the disclosure of the requested information, that HJI will approach a court of law for appropriate relief.
-
21 - 27 September 2021
The 76th session of the United Nations General Assembly (UNGA) to discuss the global COVID-19 response commenced. Along with other world leaders, South Africa’s President also participated inter alia on discussions around a global COVID-129 recovery approach.
Access the link here for more information.
-
22 September 2021
The United States Government hosted a Global COVID-19 Summit with President Biden (USA) and other world leaders. The US announced a ‘target of 70% of the world’s population’ to be vaccinated within the next year.
Access the link here and here for more information.
- Free the Vaccine wrote an Open Letter to President Biden stating that:
‘While it is commendable that your administration has procured and distributed COVID-19 vaccines to other countries in need, we cannot “donate” our way to safety. The massive global shortage of highly effective vaccines can only be addressed by increasing production. As of this week, just over 3% of people in low-income countries, many of whom are also facing devastating surges from deadly variants, have received any dose of COVID-19 vaccine.’
-
22 September 2021
The US Food and Drug Administration (FDA) approved ‘booster’ shots of Covid-19 vaccines for certain categories of patients and workers.
Access the link here for more information.
-
19 - 24 September 2021
The South African government responded to the UK government’s announcement of continued travel ‘restrictions’ including for people fully vaccinated in Africa and South America (effectively precluding people travelling from South Africa to enter the UK unless they quarantine (even where vaccinated)).
-
26 September 2021
Dr Sandile Buthelezi, Director-General of the Department of Health was officially suspended over his alleged involvement in the ‘Digital Vibes’ tender scandal following a report from the SIU. Media reports indicated that other senior health officials would also be suspended.
Access the link here for more information.
- 28 September 2021
- 29 September 2021
- 30 September 2021
-
30 September 2021
The People’s Vaccine Alliance sent an Open Letter to the Nobel Committee signed by survivors of Covid, health workers and people who have survived or lost our loved ones to Covid-19 to object to the consideration of Prof. Şahin and Dr. Türeci (of BioNTech) for a Nobel Prize, on the grounds that they have refused to share the vaccine knowledge and know-how with the developing world.
The letter stated:
‘We have nothing but respect for your scientific achievement. But we have nothing except despair for your subsequent failure to share that science with the world. Were you to agree to share your science through the WHO mRNA hub in South Africa, and its Covid-19 Technology Access Pool, and to support a comprehensive waiver of intellectual property rules, we will be the first to support your nomination for a Nobel Prize: as the individuals who used their extraordinary power to save millions of lives in every country in the world at this time of unprecedented human crisis.’
Access the link here for more information.
-
End September 2021: National Vaccine Roll-Out Status:
Total Number of Vaccines Administered in South Africa: 17 505 358
Total number of people fully vaccinated with Johnson & Johnson vaccine: 3 410 499
Total number of people fully vaccinated with Pfizer vaccine: 4 912 082
Number of people who received 1st dose of Pfizer vaccine: 8 686 228
More than 3.56 billion people worldwide received one dose of a Covid-19 vaccine (45% of the world’s population). Due to lack of access to timely vaccine supplies, Africa had the slowest vaccination rate of any continent, with just 6.8 % of the population having received at least one dose of a vaccine and less than 5% fully vaccinated by end September 2021.
Access the link here for more information.
- 5 October 2021
- 8 October 2021
- 11 October 2021
- 12 October 2021
- 13 October 2021
-
14 October 2021
A projection from Our World In Data on vaccination rates indicated that 69 countries (44 of them in Africa) were not on track to have given a first dose to 40% of their population by the end of 2021.
Which countries are on track to reach global COVID-19 vaccination targets? – Edouard Mathieu via Our World in Data COVID-19
Access the link here for more information.
-
14 October 2021
The WTO TRIPS Council meeting formally commenced to discuss the TRIPS Waiver – this is marked by global protests. The European ‘Day of Shame’ protests started on 11 October 2021 including in London (calling for the UK government to change its position and support the TRIPS waiver proposal at the WTO) and Geneva and Vienna.
Links to global protest action:
- https://www.standard.co.uk/news/uk/whitehall-british-government-coffins-campaigners-gordon-brown-b959961.html
- https://twitter.com/JustTreatment/status/1447963361925386251
- https://twitter.com/STOPAIDS/status/1447984110916485135
- US trade ambassador Kathrine Tai met with the EU ambassador to the WTO and the South Africa Minister of Trade, Industry and Competition.
- https://twitter.com/EUAmbWTO/status/1448402092457082881
- https://ustr.gov/about-us/policy-offices/press-office/press-releases/2021/october/readout-ambassador-katherine-tais-meeting-south-africa-minister-trade-industry-and-competition
The HuffPost obtained a copy of the draft text that the EU’s directorate-general for trade shared with at least one WTO member nation with whom it is negotiating. The core of the proposal, which follows a framework outlined by the EU in June, is to reiterate WTO procedures agreed to in 2001 for compelling companies to license the patents on their drugs in a public health emergency. The proposal was heavily criticized:
Access the link here for more information.
- 14 October 2021
- 14 October 2021
-
15 October 2021
The National Minister of Health, Dr Joe Phaahla, announced that the vaccination of children between the ages of 12 to 17 years of age will begin on 20 October 2021 in South Africa, with one dose of the Pfizer/BioNTech vaccine.
The South African government failed to meet a deadline for the disclosure of all vaccine contracts pursuant to an appeal lodged by the HJI using the access to information act. It also failed to disclose all relevant expert advice and information related to the prioritization of cohorts of people in the national vaccine roll out. As a result, the HJI will be approaching a court of law to compel the disclosure.
- 18 October 2021
- 19 October 2021
- 19 October 2021
-
21 October 2021
The Peoples Vaccine Alliance released a Report entitled Dose of Reality – it detailed how rich countries and pharmaceutical corporations broke their ‘vaccine promises’ to the global south.
“This unacceptable cutback makes a catastrophic situation even worse. Since the start of the pandemic, only 0.7 % of all manufactured vaccine doses have gone to low-income countries.”
- 21 October 2021
- 22 October 2021
-
22 October 2021
In an Editorial in the journal Science, the HJI and I-MAK jointly argued that the world’s Vaccine Know should be shared
“To achieve equity, intellectual property (IP) relating to COVID-19 vaccines should be temporarily waived to bolster their production. Waiving IP rights and sharing knowledge will allow middle- and low-income countries to produce their own vaccines rather than rely on donations and COVAX.”
-
24 October 2021
Namibia discontinued the use of the ‘Sputnik V’ vaccine following on from the non-approval by South Africa’s SAPHRA.
The Financial Times reported that poorer countries relying on COVAX received ‘over 16 times less’ vaccines than wealthy nations. Access the link here for more information.
-
26 October 2021
Moderna announced that it would for the first time ‘supply up to 110 million doses’ of vaccines to Africa via the African Union.
Access the link here for more information.
-
26 October 2021
The HJI and the WITS University Mandela Institute hosted the final Webinar in a series addressing the legal and policy issues arising out of the proposed WTO TRIPS waiver.
The way forward on and beyond the TRIPS waiver proposal: Recording: https://youtu.be/j3uQK-sdB4Y
- 26 October 2021
-
26 October 2021
BioNTech announced that it entered into an agreement with the Rwandan Government and Institute Pasteur de Dakar in Senegal for the construction of an mRNA vaccine manufacturing facility commencing mid-2022. Activists lament that this is too late and a ‘drop in the ocean’.
Access the link here for more information.
The People’s Vaccine Alliance responded to the announcement: “After huge public pressure, BioNTech has finally committed to manufacturing vaccines in the global south. While this is a positive development, it’s far too little, far too late from a company that has made a killing from the pandemic.”
Access the link here for more information.
-
27 October 2021
The HJI, African Alliance and People’s Health Movement held “Africa: The 5% Continent Greed, Divides and Solidarity” second webinar. DIVIDES
-
27 October 2021
US-based pharmaceutical corporation Merck announced a ‘voluntary license’ agreement with the Medicines Patent Pool (MPP) - the agreement granted MPP permission to sublicense molnupiravir to manufacturers in multiple countries to supply people in 105 low- and middle-income countries (LMICs) (where and if approved for use).
While it is the first fully transparent voluntary license covering a COVID-19 medical tool on a royalty-free basis, Doctors Without Borders/ Médecins Sans Frontières (MSF) stated that it was ‘disappointed with the limitations of the license, as well as a harmful provision that undermines the right of generic companies that sign the license to challenge patents to facilitate generic drug production’.
Access the link here for more information.
-
28 October 2021
The US government announced a $1.3 Billion investment for Global Vaccine Manufacturing in its ‘Build Back Better’ Plan
US Group Public Citizen commented on the announcement that:
“…it could support the World Health Organization and African vaccine manufacturers’ effort to build the world’s first mRNA technology transfer hub in South Africa, which would be open to manufacturers worldwide and share know-how with humanity…”.
Access the link here for more information.
-
31 October 2021
National Vaccine Roll-Out Status:
Total Number of Vaccines Administered in South Africa: 22 381 951
Total number of people fully vaccinated with Johnson & Johnson vaccine: 4 767 627
Total number of people fully vaccinated with Pfizer vaccine: 17 117 370
Number of people who received 1st dose of Pfizer vaccine: 15 239 094
Global statistics at the end of October 2021
More than 3.9 billion people worldwide received one dose of a Covid-19 vaccine (49% of the world’s population). Only 3.6% of people in low-income have received at least one dose.
Access the link here for more information.
-
31 October 2021
End of October South African Statistics:
Total number of people fully vaccinated with Pfizer vaccine (Dose 1 and Dose 2):
7 052 854
- 8 November 2021
-
9 November 2021
Biolyse/Bolivia/ Canada: Bolivian Vice-minister of Foreign Trade and Integration Benjamin Blanco, Canadian Member of Parliament Niki Ashton, the Council of Canadians, Biolyse and Knowledge Ecology International (KEI) asked that the Canadian Federal Government allow COVID-19 vaccines to be manufactured in Canada through the country's compulsory license mechanism; and that the Government of Canada support a temporary waiver on intellectual property rights on COVID-19 vaccines and medicines at the WTO.
This was accompanied by a parliamentary petition in Canada, led by the Council of Canadians and Canadian MP Niki Ashton. Access the link here for more information.
- 9 November 2021
-
10 November 2021
International medical humanitarian organisation Doctors Without Borders/Médecins Sans Frontières (MSF) delivered petition to the US Government, calling on President Biden to ensure global vaccine equity.
Johnson & Johnson announced that it entered into an agreement to provide vaccines to COVAXs Humanitarian Buffer. However, at the same time, COVAX has indicated that vaccine manufacturers are refusing to waive indemnification provisions preventing the delivery of vaccines to communities requiring humanitarian assistance (refugees mainly).
The South African Medical Association (SAMA) submitted an ‘open letter’ to the National Minister of Health, Dr Joe Phaahla calling for the right of health care workers to ‘choose their preferred vaccine booster’ and directed a number of questions to the Minister regarding the vaccine booster roll-out study for health care workers.
Ref:
- https://www.samedical.org/cmsuploader/viewArticle/1938
- Access the link here and here for more information.
The South African College of Medicine also issued a letter indicating that:
- The majority of health workers in South Africa have received the J&J vaccine as that was the only option available to them at the time. There is evidence that the one-dose J&J regimen has lower efficacy that the two-dose mRNA regimens. The CDC currently recommends that “People who received the Johnson & Johnson/Janssen COVID-19 vaccine and are 18 years and older should receive a booster shot at least 2 months after receiving their primary vaccine dose”. Without a booster of any kind, our health workers who received the J&J vaccine are less than optimally protected.
- There is evidence that immunity wanes and breakthrough infections increase over time with all of the currently available COVID-19 vaccines. This further highlights the need for a booster for South Africa’s health workforce given the time that has elapsed since the majority of health workers received the J&J vaccine as part of the Sisonke Study.
- While both homologous and heterologous boosters have been shown to increase binding and neutralising antibody titres, there is some evidence that mRNA booster produces substantially higher binding and neutralising antibody titres than a second dose of J&J vaccine in those who received a primary J&J vaccine. We acknowledge though that the data supporting heterologous boosters is currently limited but encouraging nonetheless.
- There are anecdotal reports that large numbers of health workers intend to sit out the Sisonke booster programme and wait for a Pfizer booster to become available to them in South Africa. While they wait for that to happen, they remain less than optimally protected. In our view, they should be provided with an alternative as soon as possible in order to achieve high booster vaccine coverage among this population quickly, and ahead of a potential fourth wave.
- There are reports of significant levels of Pfizer wastage at vaccine sites which could provide an avenue for NDoH to provide health workers with the Pfizer option.
- Both homologous and heterologous boosters have a potential role to play in responding to the COVID-19 pandemic going forward and this should be borne in mind in order to avoid further vaccine hesitancy.
-
10 November 2021
The Peoples Vaccine Alliance wrote to the CEO of Moderna urging Moderna to
‘to accelerate efforts to end the pandemic around the world’. The letter stated that Moderna’s vaccine has ‘so far almost exclusively been supplied to wealthy countries, while low-and middle-income countries are left without, or with long waits and high prices.’
-
11 November 2021
Moderna’s COVID-19 vaccine patent dispute with the US National Institutes of Health (NIH) intensified and US courts began adjudicating it.
The resolution of this dispute will have an impact on the first WHO mRNA Hub established in South Africa too.
- 12 November 2021
- 16 November 2021
- 16 November 2021
- 20 November 2021
- 20 November 2021
- 22 November 2021
- 23 November 2021
- 24 November 2021
- 24 November 2021
-
25 November 2021
South African and Botswanan scientists announced the discovery of a new COVID-19 variant: ‘B1.1.529’ - subsequently named Omicron.
Important Note:
Subsequently, it emerged that the variant was circulating in Europe for at least about a week before:
Access the link here and here for more information.
In response to the announcement, flights from several Southern African countries are banned from entering the Netherlands, Switzerland, Germany, France, UK, US, UAE, Canada and several other European countries, affecting all travel regionally and globally. The selective travel bans were immediately met with condemnation from the South African government, scientists and activists in South Africa, the region and abroad as well as the World Health Organisation (WHO).
- https://www.dailymaverick.co.za/article/2021-11-26-uks-knee-jerk-red-list-temporary-ban-throws-south-african-travellers-tourism-industry-and-airlines-into-a-muddle/
- https://www.bbc.com/news/uk-59424269
- http://www.dirco.gov.za/docs/2021/uk1126.htm
- https://www.democracynow.org/2021/11/29/headlines/south_africa_blasts_travel_bans_as_countries_race_to_curb_spread_of_omicron_variant
- https://www.youtube.com/watch?v=xR3eLw5CdMU
- https://www.theguardian.com/world/2021/dec/07/omicron-covid-variant-is-already-everywhere-who-says
- https://www.bbc.com/news/world-africa-59432579
–The HJI’s response to the travel ban was set out in an op ed for Al Jazeera- available here.
–The HJI’s conversation with NPR – All Things Considered – about the travel bans and vaccine apartheid is available here.
The World Health Organisation (WHO) shortly thereafter also designated the variant as a ‘variant of concern’, named Omicron:
BioNTech, Moderna and Johnson & Johnson were reported to have ‘started working on vaccines that specifically target Omicron in case their existing shots are not effective against the new coronavirus variant’
- https://www.reuters.com/business/healthcare-pharmaceuticals/moderna-can-take-months-ship-omicron-specific-vaccine-ceo-says-cnbc-2021-11-29/
- https://www.bloomberg.com/news/articles/2021-11-29/biontech-is-working-on-an-omicron-version-of-its-covid-shot?utm_source=Airfinity&utm_campaign=d6238a5789-EMAIL_CAMPAIGN_2021_11_30_12_14&utm_medium=email&utm_term=0_41a531e556-d6238a5789-464791401
Omicron and the 12th WTO Ministerial Conference:
The 12th MC was postponed indefinitely due to the new variant and also travel restrictions. Globally, activists continued to call for an urgent resolution and passing of the TRIPS Waiver:
https://twitter.com/peoplesvaccine/status/1465650420899094529
https://www.twn.my/title2/intellectual_property/info.service/2021/ip211108.htm
Also:
200 million workers called for a TRIPS Waiver : https://www.itfglobal.org/en/news/global-unions-join-biden-eu-parliament-in-call-urgent-waiver-vaccine-patents-tackle-dangerous and https://twitter.com/PSIglobalunion/status/1465312458575392775
https://ourworldisnotforsale.net/MC12
- Norway changed its stance on the TRIPS WAIVER by indicating support: https://www.vg.no/nyheter/utenriks/i/66V8z3/norge-snur-vil-la-vaksine-oppskriftene-deles-med-fattige-land
- The US President indicated renewed US support for a Covid-19 ‘vaccine waiver’, but media reports indicated otherwise – stating that ‘Documents Reveal Biden Admin Not Fighting for a Covid Vaccine Patent Waiver, Despite Public Statements’:
https://inthesetimes.com/article/biden-omicron-wto-trips-waiver-intellectual-property-patents
- In Canada, the Leader of Canada’s New Democratic Party called on the Prime Minister to take a position on the TRIPS waiver so that more countries can produce vaccines locally.
- 27 November 2021
-
27 November 2021
The South African Government noted the announcements made by the UK, Canada, USA and several other countries in response to the identification of Omicron (a new variant) - to institute’ temporary travel restrictions’ on Southern African countries.
The Department of International Relations and Cooperation (DIRCO) also expressed concerns about the damage caused by ‘travel restrictions’ to families, businesses, and the travel and tourism industries.
South African scientists, health experts, civil society groups and others, also condemned the restrictions as ‘racist’ in its selective scope.
- Ref: http://www.dirco.gov.za/docs/2021/covid-19_1127.htm
- Ref screenshots:
- 28 November 2021
- 28 November 2021
-
28 November 2021
The South African Department of International Relations and Cooperation (Dirco) announced that flights from South Africa to the UK would resume on 30 November 2021 but with stringent restrictions, conditions and quarantine periods.
Info received from @ukinsouthafrica.
— Clayson Monyela (@ClaysonMonyela) November 28, 2021
- @British_Airways will resume direct flights to #SouthAfrica from Tuesday (30 Nov) with the 1st flight to London on W/day. It'll be 1 daily flight to Cape Town & ORTIA.
- Virgin flies 3 times a week into ORTIA. They haven't stopped theirs. -
30 November 2021
Aspen announced that it had signed a ‘non-binding’ term sheet with Johnson & Johnson, that will form the basis for ‘negotiations of a definitive agreement on the manufacture and sale of an Aspen-branded COVID-19 vaccine throughout Africa.’
This could provide Aspen the rights to:
- ‘Manufacture a finished SARS-CoV-2 COVID-19 vaccine product,’ but from ‘COVID-19 drug substance supplied by Johnson & Johnson’; and
- ‘Sell the finished form vaccine, to be launched and branded as “Aspenovax”, to public sector markets in Africa…’
-
30 November 2021
End November 2021: OMICRON
Omicron risk has caused 68 countries to impose travel bans, affecting 40.4% of the world’s population. Airfinity reported that:
‘Several countries have imposed travel restrictions since 26th November 2021. The EU countries have largely imposed a travel ban from the 6 African countries; Switzerland and Greenland allow travel for vaccinated or fully recovered passengers. Morocco and Japan have taken strong measures by imposing a complete travel ban from all countries. With 32% of the world’s countries imposing a travel ban for all 6 African countries listed, 40.4% of the world’s population is affected’
End November 2021: National Vaccine Roll-Out Status:
Total Number of Vaccines Administered in South Africa: 25 619 891
Total number of people fully vaccinated with Johnson & Johnson vaccine including Sisonke Trial 1:
6 116 065
Total number of HCWs who received a second dose of the Johnson & Johnson Vaccine under Sisonke Trial 2: 154 030
Total number of people fully vaccinated with Pfizer vaccine (Does 1 and Dose 2):
8 358 689
Number of people who received 1st dose of Pfizer vaccine: 17 106 172
Access the link here and here for more information.
Global statistics at the end of November 2021
More than 4.2 billion people worldwide received one dose of a Covid-19 vaccine (54.4% of the world’s population). But, of this, only 6% of people in low-income countries received at least one dose.
Access the link here for more information.
-
30 November 2021
The Financial Times released its investigative report on the Pfizer vaccine entitled: “The inside story of the Pfizer vaccine: ‘a once-in-an-epoch windfall’”
The Financial Times spoke to ‘‘more than 60 people involved in the vaccine process, including current and former Pfizer employees and government officials across the globe, to lift the veil on how the company that has contributed so much to saving the world from Covid has also ensured it is such a lucrative business. They are all grateful for a safe and effective vaccine. But many question if the balance of power has tipped too far in Pfizer’s favour’.
-
29 November 2021
Global Nurses United, a coalition of nurses' union from around the world filed a complaint with the United Nations alleging human rights violations by the European Union, the United Kingdom, Norway, Switzerland, and Singapore during the Covid-19 pandemic. The health care workers call a temporary waiver of intellectual property protections for COVID-19 vaccines and treatments.
Ref: Nursing unions around the world called for UN action on Covid vaccine patents
Access the link here for more information.
- Germany, UK, US, Norway and Switzerland face legal review of their actions –domestically- in “prolonging the pandemic”.
Access the link here for more information.
-
3 December 2021
The Minister of Health Dr Joe Phaahla and Deputy Minister of Health Dr Sibongiseni Dhlomo, convened a virtual media briefing on government efforts in the fight against COVID-19 and the national vaccination rollout programme. The Minister highlighted that:
- There has been a steep increase in Covid-19 cases observed in Tshwane spreading into the rest of Gauteng, Johannesburg, Ekurhuleni and Sedibeng due to newly discovered Covid-19 variant, Omicron.
- They expressed disappointment at countries that imposed travel bans for Southern African countries following the discovery of Omicron.
- 8 December 2021
- 9 December 2021
- 10 December 2021
- 14 December 2021
- 15 December 2021
- 16 December 2021
- 17 December 2021
-
17 December 2021
The Minister of Health Dr Joe Phaahla and Deputy Minister of Health Dr Sibongiseni Dhlomo, convened a virtual media briefing on government efforts in the fight against COVID-19 and the national vaccination rollout programme. The Minister highlighted that:
All South African provinces entered the 4th wave of Covid-19 and the Gauteng province reported the highest number of new cases per day.
- 21 December 2021
- 22 December 2021
- 22 December 2021
-
23 December 2021
The Department of Health announced the implementation of Covid-19 vaccine booster doses. The Circular indicated:
- Johnson & Johnson (second shot): for anyone who received their first dose of J&J at least 2 months prior
- Pfizer (third shot): for anyone who received their second dose of Pfizer at least 6 months prior, commencing with those over 18 years old.
Note: The department did not address the issue of heterologous boosters (in other words, for example, using a Pfizer second shot for someone who received a J&J first shot).
- 23 December 2021
- 29 December 2021
- 30 December 2021
2022
- 14 January 2022
- 14 January 2022
-
14 January 2022
The Minister of Health Dr Joe Phaahla and Deputy Minister of Health Dr Sibongiseni Dhlomo, convened a virtual media briefing on government efforts in the fight against COVID-19 and the national vaccination rollout program. The minister highlighted the following:
- Overall decline in hospital admissions
- Gauteng Province is exiting the 4 wave
- Vaccination has increased but remains below 100 000 vaccinations per day nationally
- Issue of vaccine mandates currently discussed by government and further announcements to be made in the future.
- 19 January 2022
- 25 January 2022
- 29 January 2022
-
30 January 2022
More than 100 civil society organisations including the HJI wrote an open letter to WHO Director General calling for the WHO to expedite the finalisation and release of ‘self-testing guidelines’ for COVID-19 that includes a recommendation favouring widespread access to self-testing. The letter highlighted the following reasons for access to diagnostics:
- People have a fundamental right to ‘know their status’
- Self-testing is critical to prevent onward transmission and to empower individuals to protect their families and communities
- Self-testing is a necessary tool to enable rapid linkage to care and initiation of outpatient treatment to prevent hospitalisation and death, especially among those at high risk of disease progression
Ref:
https://oneill.law.georgetown.edu/publications/open-letter-to-the-world-health-organization/
South Africa: end January vaccination statistics
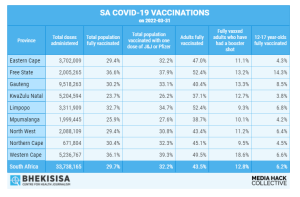
Total number of vaccines administered: 29 906 633
Total no of adult individuals vaccinated: 18 542 938 or 46.59% of adult population
(1 dose J&J and only 1 dose Pfizer)
Total number of J&J vaccines administered: 7 314 423
- Total number of first dose of vaccine administered: 6 947 007
- Total number of (second) ‘booster’ doses administered to HCWs as part of ‘Sisonke 2’: 370 416
Total number of Pfizer vaccines administered: 22 567 267
- Total number of first doses administered: 12 664 645
- Ref: https://sacoronavirus.co.za/latest-vaccine-statistics/
Global: end January vaccination statistics
More than 10.08 billion people worldwide have received at least one dose of COVID-19 vaccine (61.1% of the world’s population) but only 10% of people in low-income countries received at least one dose.
- 31 January 2022
-
04 February 2022
Afrigen (a company in South Africa) announced that it had used publicly available data to produce its own vaccine candidate - the first to be based on a widely used vaccine without the assistance and approval of the developer – in this case Moderna. Afrigen is part of the WHO – SA mRNA Hub based in South Africa.
Access the link here for more information.
- 04 February 2022
- 08 February 2022
-
09 February 2022
The British Medical Journal (BMJ) reported that it had discovered evidence that an organisation called kENUP allegedly tried to undermine the WHO’s efforts to bring vaccine manufacturing capacity to Africa by actively discouraging stakeholders from participating in the work of the WHO- SA mRNA Hub.
- The BMJ disclosed that the kENUP Foundation, a consultancy hired by BioNTech, had claimed that WHO – SA mRNA Hub was ‘unlikely to be successful and will infringe on patents’.
- The BMJ also reported based on documents it had seen, that kENUP was instead ‘promoting BioNTech’s proposal to ship mRNA factories housed in sea containers from Europe to Africa, initially staffed with BioNTech workers, with a proposed new regulatory pathway to approve the vaccines made in these factories’ and that kENUP had been influencing ‘South African manufacturers not to get involved’ in the work of the Hub, calling for the project to be ‘terminated immediately’.
Access the link here for more information.
In response to these worrying revelations, the People’s Vaccine Alliance issued a statement condemning the actions and conduct of kENUP and BioNTech:
Access the link here for more information.
-
10 February 2022
The Annual State of the Nation Address (SONA, 2022) was delivered by President Cyril Ramaphosa. IN respect of the Vaccine Programme in SA, he stated that there:
- Had been increased efforts to develop Africa’s ability to manufacture vaccines;
- Had been increased local capability to produce ventilators, hand sanitisers, medical masks, gloves and therapeutic drugs and anesthetics.;
- Have administered more than 30 million doses of COVID-19 vaccines (nearly, 42% of all adults)
Access the link here for more information.
-
11 February 2022
South African Civil Society Organisations (CSO) including the HJI wrote an open letter to Moderna regarding the patents it had filed, and which was granted in South Africa, in 2021, that pose a legal risk for the optimal functioning of the WHO- SA mRNA Hub.
-
The letter requested that Moderna also :
- Withdraw and abandon all patents and patent applications in South Africa related to the Moderna Covid-19 mRNA vaccine technology.
- Immediately lend technical assistance to the mRNA Hub to guarantee its success within an expedited period.
Access the link here for more information.
Dr Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization (WHO) accompanied by a WHO delegation, SA government officials and Ministers, as well as the Belgian Minister of Development Cooperation and Urban Policy visited the WHO – SA mRNA Hub in Cape Town. As part of the WHO DG’s visit to South Africa, he met with several civil society organisations based in SA who requested an opportunity to discuss several aspects of vaccine, treatment and testing equity for Africa.
-
-
16 February 2022
Given significant delays in negotiating the text of the TRIPS WAIVER, global Civil Society Organisations wrote an Open Letter to WTO Director General Dr. Ngozi Okonjo-Iweala. The letter called for a credible WTO’s response to the pandemic which is not only limited to vaccines.
The letter stated: “Any credible waiver outcome must also equally cover the medical products essential to control COVID-19 and especially vaccines, therapeutics and diagnostics, including their materials and components. Addressing access to therapeutics and diagnostics must not be delayed.”
- 17 February 2022
- 17 - 18 February 2022
-
18 February 2022
The WHO announced the first set of countries in Africa that will be considered the ‘spokes’ of the WHO – SA mRNA Hub.
The spokes will benefit from technology transfer (needed to make mRNA vaccines in Africa) and they include: Kenya, Egypt, Senegal, Tunisia, Nigeria and South Africa.
-
21 February 2022
The South African National Department of Health published certain changes to the vaccine programme’. Circular 2 of 2022 introduced changes to dosing intervals and included the approval and introduction of ‘heterologous boosting’.
The changes included:
As at 23rd February 2022: re the Pfizer programme:
– the interval between the first and second doses of the Pfizer vaccine was reduced from 42 days to 21 days. The interval of the booster dose was reduced from 180 days to 90 days after receiving the second dose of the vaccine.
– Individuals over the age of 18 years who received two doses of the Pfizer vaccine were thus eligible to receive a booster dose of the same vaccine or a booster dose of the J&J vaccine (also within 90 days of receiving the second of the two doses of the Pfizer vaccine).
As at 21 February 2022: re the Johnson & Johnson programme:
- the approval of heterologous booster doses i.e. booster doses of a different vaccine to that which was administered as the primary series – individuals older than 18 years of age who received one dose of J&J were eligible to receive a booster dose of the same vaccine or a booster dose of the Pfizer vaccine after an interval of 60 days.
-
22 February 2022
The Health Justice Initiative approached the South African courts for the disclosure of all vaccine manufacturer contracts and agreements, for Covid-19.
- The case was lodged after multiple access to information requests were either ignored or not complied with.
- We argued in legal papers that the public has a right to know what the terms and conditions of each contract are, and that the onus is on the National Department of Health (The Minister acting for the SA government) to disclose the names of all the contracting parties, and to join them to the proceedings as Respondents.
-
22 February 2022
The Health Justice Initiative’s 2021 – 2022 “Vaccine Supplies Summary” (No. 4) sheet showed the 12month supply summary for vaccines. HJI found that according to information available to it as at mid-February 2022, approximately:
- 1/3 of deliveries on the South African J&J contract and about 1.8 million vaccines from the South African Pfizer contract are outstanding.
- Further that key policy decisions on vaccine selection and use would have to be urgently taken by the SA government.
-
February 2022
End of the month vaccine statistics
-
- Total number of vaccines administered to children aged 12 –17 years: 1 597 820
- Total Number of booster vaccines administered (Pfizer and J&J): 1 104 335
- Total number of vaccines administered to immunosuppressed people: 19 706
- Total Number of HCWs vaccinated under the Sisonke Programme:
- Sisonke 1st dose: 499 946;
- Sisonke 2nd dose: 240 878
- Access the link here for more information.
South Africa
Total Number of Vaccines Administered in South Africa: 31 544 594
Global Vaccine Statistics
By end February 2022: more than 62.8% of the world population received at least one dose of a COVID-19 vaccine; more than 10 billion vaccine doses were administered globally, but only 12.6% people in low-income countries received at least one dose by then.
Access the link here for more information.
-
-
4 March 2022
Media Briefing by the Department of Health: The Minister of Health highlighted that:
- Omicron remained the most dominant variant in SA.
- Hospitalisation decreased by 11% and 72% of admissions were patients who were unvaccinated.
- The rate of infections had not declined steadily as recurring spikes have been observed in several districts
-
11 March 2022
The Department of Health published amendments to the national Covid-19 vaccine programme. Circular 4 of 2022 introduced changes to dosing intervals and included the approval and introduction of ‘heterologous boosting’.
The changes included:
As at 14 March 2022: Pfizer programme:
- Individuals over the age of 18 years who received two doses of the Pfizer vaccine are eligible to receive an additional (2nd) booster dose of the same vaccine or a booster dose of the J&J vaccine (within 90 days of receiving the first booster doses of the Pfizer vaccine).
As at 14 March 2022: Johnson & Johnson programme:
- Individuals older than 18 years of age who received one dose of J&J are eligible to receive an additional (2nd) booster dose of the same vaccine or a booster dose of the Pfizer vaccine after an interval of 90 days.
-
14 March 2022
The National State of Disaster was extended to 15 April 2022.
Access the link here for more information.
-
16 March 2022
TRIPS WAIVER negotiations:
A ‘leaked document’ was published– indicating that a proposed ‘text’ for a negotiated settlement, existed.
Many commentators argue that the leaked text is a ‘watering down’ of the TRIPS Waiver; represents an inadequate and ‘bad deal’; and should not be signed onto by countries in the global south and others.
The HJI’s library of the ‘leaked text’ is available here.Access the link here and here for more information.
Wide-spread criticism from civil society groups, economists and experts from across the world followed, which highlighted the following key shortcomings of the leaked text:
- The leaked text contains proposals that are very narrow, and it focuses only on vaccine patents (thus, not all Intellectual Property restrictions):
- The leaked text covers vaccines only, not diagnostics and treatments:
- The leaked text includes onerous TRIPS + conditions for compulsory licensing
Also, that the process being followed is of serious concern and attempts to steamroll the WTO and especially global south members sets a negative precedent for the future:
Prof Joseph Stiglitz (Nobel Laureate), Prof Jayati Ghosh and Mr Peter Kamalingin wrote a letter to President Ramaphosa urging him to reject the deal.
They wrote: ‘We strongly support South Africa not agreeing to this proposal. We are keen to work with you as you lead the world to obtain a useful and meaningful outcome that facilitates diversification and expanded production and supply. Like civil society groups around the world, we believe a bad deal is worse than no deal. We want to work with you to support an outcome at WTO that will make a difference in battling COVID. The leaked text fails that test.’
Access the link here for more information.
South African academics and researchers also wrote to President Ramaphosa, to urge him to reject the ‘deal’. They wrote: ‘Unfortunately, the draft text is defective both in its fundamentals and in its details. In terms of fundamentals, the draft text fails to deliver meaningful progress even on vaccines, its stated, exclusive focus.’
Access the link here for more information.
33 Civil society organisations and 134 individuals in South Africa wrote to Minister Patel, to urge him to reject the ‘bad deal’ contained in the leaked text. They called on Minister Patel and government to “NOT endorse this very bad deal’’ and to “insist that the negotiations on the text do not side-line other global south countries, as the precedent that sets, is to favour rich nations.’’
Access the link here for more information.
- 22 March 2022
-
25 March 2022
Media Briefing by the Department of Health
-
March 2022
End of the month statistics
South Africa
- Total Number of Vaccines Administered in South Africa: 31 544 594
- Ref: https://sacoronavirus.co.za/latest-vaccine-statistics/
- Data on the vaccination of adults and children is available at: https://bhekisisa.org/resources/2021-07-12-covid-19-vaccinations-how-are-the-provinces-doing/
Global Statistics
- By end March 2022: 64.5% of the world population had received at least one dose of a COVID-19 vaccine.
- Only 14.5% of people in low-income countries have received at least one dose of a vaccine.
- Total Number of Vaccines Administered in South Africa: 31 544 594
-
4 April 2022
SOUTH AFRICAN STATE OF DISASTER DECLARATION is lifted
- The declaration of the National State of Disaster was issued on 15 March 2020 under South Africa’s Disaster Management Act.
- For 750 days, South Africa has been in a ‘National State of Disaster’. At midnight on 4 April 2022 the declaration was lifted.
- Statement by President Cyril Ramaphosa on the termination of the National State of Disaster in response to the Covid-19 pandemic: Reference:
According to the Statement by President Cyril Ramaphosa:
- From midnight 4 March 2022, the COVID-19 pandemic in South Africa will be managed in terms of the National Health Act – for which draft Health Regulations have been published for public comment (until 16 April 2022) (See link here for more information.
- Because the Disaster Management Act provides that certain elements of the regulations may remain in place for a limited period for ‘post-disaster recovery and rehabilitation’, ‘some transitional provisions will remain in place for a period of 30 days after the termination of the National State of Disaster to ensure essential public health precautions and other necessary services are not interrupted while the new regulations in terms of the National Health Act come into effect’ (related to inter alia wearing of face masks, gathering limits, travel entry requirements, social relief grants).
- The President also stated that ‘one last measure’ will continue despite the lifting of the State of Disaster: The COVID-19 Vaccine Injury No-Fault Compensation Scheme, will continue after the National State of Disaster ends and only terminated ‘once it has achieved its purpose’. This ‘purpose’ has not been explained.
- The HJI has made a previous submission on the compensation scheme, available here.




